
High performance gas analyzers for
Power Industry
GasEye analyzers can be applied in several places in a power plant starting from a coal silo (CO measurement), through boiler optimization (CO+O2+CH4+H2O) to emission control (NO, NO2, NH3, SO2, SO3) and CEMS (HCl, NH3, HCHO, HF). Both in-situ as well as extractive analysis is possible.
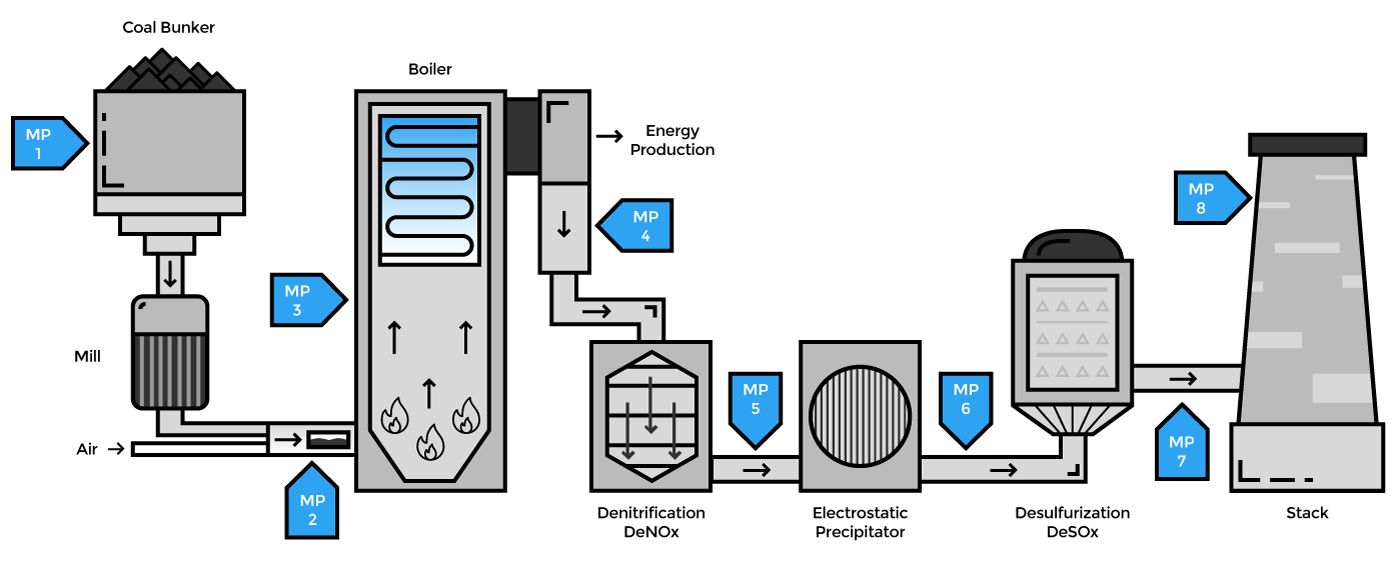
Coal Silo (CO)
Possible solutions:
Coal mill inerting system (O2)
Possible solutions:
Optimization of combustion (O2, CO, CH4, H2O)
Possible solutions:
Upstream the denitrification stage (NO, NH3)
Selective Catalytic Reduction (SCR) deNOx installations are common for large scale combustion plants like coal fired power utilities. Nitrogen oxides (NOx) formed in combustion processes are efficiently reduced to water and nitrogen in the SCR process. Ammonia (NH3) or urea (CO(NH2)) is introduced to the flue gases upstream of a heterogeneous catalyst where the reduction takes place. Depending on the amount of dust, type and concentration of acidic gas components in the flue gas, the SCR process is normally operated in the temperature range of 300 °C – 400 °C. One GasEye cross-duct sensor can be used to control the ammonia concentration in-situ directly after the high temperature reaction, enabling real-time control of the exact dosage of NH3.
Possible solutions:
After denitrification, before electrostatic filter (NH3, NO)
The GasEye cross duct NH3 offers a sensitive measure of the ammonia slip with a short response time. Direct measurement of the NO concentration before and after the denitrification stage enables direct supervision of the efficiency of the deNOx process.
Possible solutions:
Electrofilter protection (CO)
Possible solutions:
De-sulfurization (SO2, SO3)
Possible solutions:
CEMS
In 2005, the European standard EN 14181 was introduced, which provides a formal quality assurance procedure to be applied to CEMS installations.
The EN14181 standard defines three Quality Assurance Levels – QAL1, QAL2 and QAL3 – and an Annual Surveillance Test (AST). In general, a QAL2 certification is required, which means regular in-situ calibration of the CEMs equipment.